HER2-positive breast cancer used to be one of the most dangerous types of breast cancer. About 15% to 20% of all breast cancer cases fall into this category, and for years, patients faced high risks of recurrence and spread. But over the last 25 years, a revolution in treatment has changed everything. Today, HER2-positive breast cancer is no longer a death sentence-it’s a manageable condition for many, thanks to a new class of drugs designed to attack cancer at its source.
What Makes HER2-Positive Breast Cancer Different?
HER2 stands for human epidermal growth factor receptor 2. It’s a protein on the surface of breast cells that helps them grow. In healthy cells, HER2 is present in normal amounts. But in HER2-positive breast cancer, the gene that makes this protein gets overactive, causing too many HER2 proteins to be made. This floods the cancer cells with growth signals, making them multiply fast and spread aggressively.
Doctors test for HER2 status using a biopsy. Two tests are used: IHC (immunohistochemistry) and FISH (fluorescence in situ hybridization). If the result shows strong HER2 overexpression (3+ on IHC or gene amplification on FISH), the cancer is classified as HER2-positive. This isn’t just a label-it determines exactly which drugs will work.
The First Breakthrough: Trastuzumab and Beyond
The game-changer came in the late 1990s with trastuzumab (Herceptin), the first drug built to lock onto HER2 proteins and block their signals. It didn’t just slow cancer-it shrank tumors and saved lives. Today, trastuzumab is still the backbone of treatment, used in early-stage and advanced cancer alike.
But trastuzumab alone isn’t enough for most people. Now, it’s almost always paired with another drug called pertuzumab (Perjeta). Together, they block HER2 in two different ways, making it harder for cancer to escape. This combo, known as dual HER2 blockade, is now standard for larger tumors before surgery (neoadjuvant therapy) and for advanced disease.
There are also biosimilar versions of trastuzumab-Kanjinti, Ogivri, Ontruzant, Herzuma, and Trazimera-that work the same way but cost less. They’ve made treatment more accessible without sacrificing effectiveness.
Antibody-Drug Conjugates: Smart Bombs for Cancer Cells
The biggest leap forward in recent years has been antibody-drug conjugates, or ADCs. These are like guided missiles: a monoclonal antibody locks onto HER2, and once attached, it delivers a powerful chemotherapy drug directly into the cancer cell. This means more damage to the tumor-and less harm to healthy tissue.
The first ADC was T-DM1 (Kadcyla), which combines trastuzumab with a chemo agent called emtansine. It became the go-to second-line treatment after trastuzumab and pertuzumab stopped working.
But even T-DM1 has been surpassed by trastuzumab deruxtecan (T-DXd, brand name Enhertu). This newer ADC carries a chemo payload five times stronger than T-DM1’s. In the DESTINY-Breast03 trial, T-DXd cut the risk of cancer worsening or death by 72% compared to T-DM1. It’s now the preferred second-line treatment for metastatic HER2-positive breast cancer.
T-DXd also works in a new group called HER2-low. These are cancers that don’t qualify as HER2-positive but still have some HER2 protein (IHC 1+ or 2+/FISH-negative). Before T-DXd, these patients had no HER2-targeted options. Now, they do. This has expanded the number of people who can benefit from these drugs from 15-20% to over 50% of all breast cancer cases.
Small Molecule Inhibitors: Fighting Cancer Inside the Cell
While antibodies work outside the cell, tyrosine kinase inhibitors (TKIs) slip inside and block HER2 signaling from within. These are pills taken daily, not infusions. Lapatinib (Tykerb), neratinib (Nerlynx), and tucatinib (Tukysa) are the main ones.
Tucatinib stands out because it’s the only TKI proven to cross the blood-brain barrier. Brain metastases were once a nightmare for HER2-positive patients-trastuzumab couldn’t reach them effectively. But tucatinib, when combined with trastuzumab and capecitabine, improved survival by nearly five months in patients with brain tumors. That’s a huge win.
Neratinib is often used after trastuzumab to prevent recurrence in early-stage cancer, but it comes with a tough side effect: severe diarrhea. Most patients take loperamide (Imodium) prophylactically, starting at the first sign of loose stools, sometimes up to 16 mg a day. Still, some can’t tolerate it.
What About Side Effects? It’s Not All Easy
These drugs are powerful, but they come with unique risks. Trastuzumab and pertuzumab can weaken the heart. About 2-7% of patients develop heart failure during treatment. That’s why doctors check heart function with echocardiograms before and every three months during therapy.
T-DXd carries a boxed warning for interstitial lung disease (ILD) or pneumonitis. Around 10-15% of patients experience some form of lung inflammation. It’s rare but serious-some cases are fatal. Patients are told to report new coughs, shortness of breath, or fever right away.
Thrombocytopenia (low platelets) and liver enzyme spikes are common with T-DM1. Margetuximab (Margenza), a newer antibody, can cause infusion reactions and fatigue.
Patients often say the side effects are less brutal than traditional chemo-no constant vomiting or hair loss-but the new ones are sneaky. One woman on a support forum said she was terrified her cough meant lung cancer, until her oncologist confirmed it was just T-DXd. Another said the daily diarrhea from neratinib forced her to quit, even though she knew it might prevent recurrence.
How Treatment Is Sequenced Today
There’s no one-size-fits-all plan, but a typical path looks like this:
- Early-stage (before surgery): Trastuzumab + pertuzumab + chemo for 3-6 months, followed by surgery and a full year of trastuzumab.
- Early-stage (after surgery): Trastuzumab for one year. Sometimes neratinib is added for a year after to reduce recurrence risk.
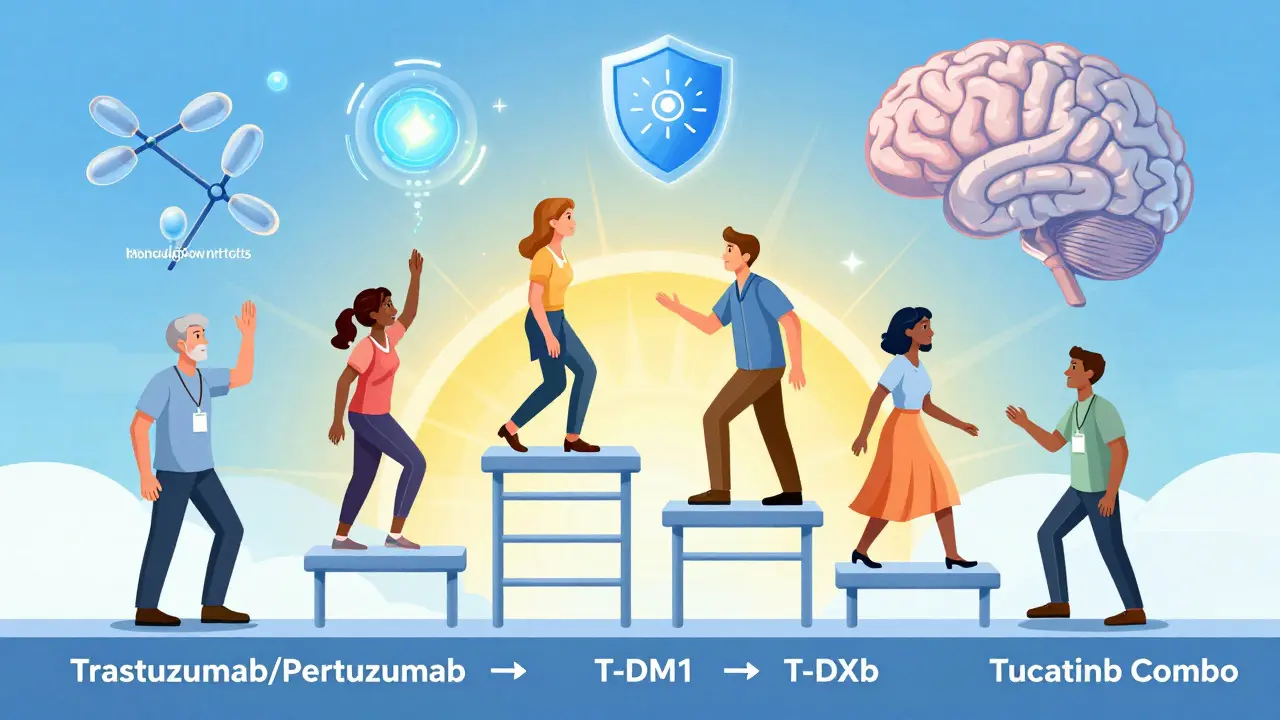
- Metastatic (advanced): First line: trastuzumab + pertuzumab + taxane chemo. Second line: T-DM1. Third line: T-DXd or tucatinib + trastuzumab + capecitabine. If T-DXd fails, newer options like bispecific antibodies or clinical trials are explored.
Doctors now think of treatment like a ladder. You start with the most effective, best-tolerated combo. When it stops working, you move up. The goal isn’t just survival-it’s quality of life.
The Future Is Already Here
The pipeline is packed with new ideas. Over 150 clinical trials are active. Bispecific antibodies like Zanidatamab can grab HER2 and another receptor at once, making cancer cells more vulnerable. Early results show 35-45% response rates in patients who’ve tried everything else.
Researchers are also testing T-DXd with immunotherapy drugs like pembrolizumab, hoping to wake up the immune system to help fight cancer. And there’s even talk of treating HER2-ultralow cancers (IHC 0 with faint staining)-a group that could include up to 70% of all breast cancer patients.
Cardiotoxicity is being tackled too. A 2023 study proposed a new risk model that looks at age, blood pressure, and heart history to predict who’s most at risk. That way, high-risk patients can get heart-protecting drugs before starting HER2 therapy.
Cost remains a hurdle. T-DXd can run over $17,000 a month in the U.S. Biosimilars have helped bring down trastuzumab prices, but newer ADCs still carry premium tags. Access isn’t equal everywhere.
What Patients Should Know
If you’re diagnosed with HER2-positive breast cancer, ask:
- Is my cancer truly HER2-positive? Double-check the test results.
- Am I eligible for dual blockade (trastuzumab + pertuzumab)?
- What’s my risk for brain metastases? If high, tucatinib may be part of your plan.
- What are the side effect risks for each drug? Know the signs of lung, heart, or gut issues.
- Are there clinical trials I should consider?
Subcutaneous options like Phesgo (a mix of trastuzumab and pertuzumab given as a shot under the skin) have cut infusion times from 90 minutes to under 10. That’s life-changing for people juggling work, kids, or long commutes.
HER2-positive breast cancer is no longer a worst-case scenario. It’s a condition with multiple, powerful tools to control it. The drugs keep getting better, smarter, and more precise. For many, the future isn’t just longer-it’s livable.
Is HER2-positive breast cancer curable?
In early stages, HER2-positive breast cancer can often be cured with surgery, chemotherapy, and targeted therapies like trastuzumab. For many, the cancer doesn’t come back after a year of treatment. In advanced stages, it’s usually not curable, but it can be controlled for years with newer drugs like T-DXd and tucatinib. Survival rates have improved dramatically-many patients live five, ten, or more years with good quality of life.
Can HER2 status change over time?
Yes. HER2 status can change, especially after treatment. A tumor that was HER2-positive at diagnosis might become HER2-negative if it spreads or resists therapy. That’s why doctors may retest a biopsy from a new tumor site. Sometimes, a tumor that was HER2-negative becomes HER2-positive after treatment. This is why personalized, ongoing testing matters.
What’s the difference between HER2-positive and HER2-low?
HER2-positive means the cancer has high levels of HER2 protein (IHC 3+ or gene amplified). HER2-low means low but detectable levels (IHC 1+ or 2+ with no gene amplification). Before 2022, HER2-low wasn’t treated with HER2-targeted drugs. Now, T-DXd is approved for both HER2-positive and HER2-low metastatic breast cancer, making it the first treatment to work across both groups.
Why do some HER2-targeted therapies cause heart problems?
HER2 proteins aren’t just on cancer cells-they’re also on heart muscle cells. Drugs like trastuzumab block HER2 signaling, which helps kill cancer but can also weaken heart function over time. That’s why heart monitoring is required. The risk is higher if you already have heart disease, high blood pressure, or are older. Newer drugs are being designed to avoid this, and heart-protecting medications can be used alongside.
How do I know if my treatment is working?
Your doctor will use scans (CT, MRI, or PET) to check tumor size, blood tests to monitor markers like CA 15-3, and your symptoms. For early-stage cancer, the goal is no signs of recurrence after treatment. For metastatic cancer, stable disease or shrinking tumors mean the treatment is working. If tumors grow or new ones appear, it’s time to switch to the next line of therapy.
Are there oral options for HER2-targeted therapy?
Yes. Tyrosine kinase inhibitors like lapatinib, neratinib, and tucatinib are pills taken daily. They’re used mainly for advanced disease or after other treatments fail. They’re convenient but come with side effects like diarrhea, fatigue, and liver changes. Subcutaneous injections like Phesgo are also faster than IV infusions but aren’t fully oral.
What’s next after T-DXd stops working?
If T-DXd stops working, your doctor may recommend clinical trials. Options include bispecific antibodies like Zanidatamab, new ADCs in development, or combinations with immunotherapy. Some patients get tucatinib-based regimens if not used before. There are over 150 active trials exploring next-gen HER2 therapies, so there’s almost always a path forward.

Jon Paramore
December 22, 2025 AT 07:31HER2-positive breast cancer treatment has evolved into a precision medicine masterpiece. Dual blockade with trastuzumab and pertuzumab is now standard-of-care in neoadjuvant settings, with pCR rates exceeding 60% in some cohorts. T-DXd’s DESTINY-Breast03 data redefined second-line therapy-72% reduction in progression risk is unprecedented. The real breakthrough? Expanding efficacy to HER2-low (IHC 1+ and 2+/FISH-) patients, which now covers over half of all breast cancers. This isn’t incremental progress-it’s a paradigm shift.
Swapneel Mehta
December 23, 2025 AT 18:00This is one of the most hopeful medical stories I’ve read in years. It’s incredible how science turned a death sentence into something manageable. I hope more people get access to these treatments, especially in places where diagnostics are still limited.
Jason Silva
December 25, 2025 AT 05:31They say these drugs save lives… but have you seen the side effects? ILD? Heart failure? And T-DXd costs $17k a month? 😏 This isn’t medicine-it’s a profit machine disguised as hope. Big Pharma doesn’t care if you live, they care if you keep paying. They’re even pushing HER2-low to expand the market. 🤔
mukesh matav
December 26, 2025 AT 18:12Thank you for laying this out so clearly. I’ve been following this field for years and even I learned a few new things about the sequencing. The part about tucatinib crossing the blood-brain barrier was especially enlightening.
Peggy Adams
December 28, 2025 AT 07:46I read this whole thing and honestly? I’m just tired. Why do we need so many drugs? Can’t we just fix the root cause? Also, why is everything so expensive? I’m not even sick and I’m exhausted just reading this.
Sarah Williams
December 29, 2025 AT 06:28For anyone newly diagnosed: you’re not alone. These treatments work. Side effects suck, but they’re manageable. Talk to your oncology team. Ask about clinical trials. And please, don’t let fear silence you. There’s real hope here.
Christina Weber
December 30, 2025 AT 13:07There is a grammatical error in the section discussing HER2-low: "HER2-low means low but detectable levels (IHC 1+ or 2+ with no gene amplification)." The phrase "with no gene amplification" is a nonrestrictive clause and requires a comma before it. Additionally, "IHC 1+ or 2+" should be hyphenated as "IHC 1+ or 2+" when used adjectivally. Precision matters in medical communication.
Cara C
January 1, 2026 AT 11:57It’s amazing how far we’ve come. I’ve seen patients go from being told they have months to live to celebrating five-year anniversaries. The emotional toll is real, but the science is giving people back their lives. Keep asking questions, keep pushing for access, and don’t let anyone tell you this isn’t progress.
Dan Adkins
January 2, 2026 AT 17:20While the advancements in targeted therapy are commendable, one must not overlook the systemic disparities in access. In many developing nations, the infrastructure required for accurate HER2 testing remains absent. Furthermore, the exorbitant cost of ADCs renders them inaccessible to the majority of the global population. The ethical imperative to democratize these therapies cannot be overstated.
Erika Putri Aldana
January 3, 2026 AT 05:43So we’re just gonna keep making more drugs instead of fixing the environment? 😒 Cancer is caused by toxins, stress, bad food… not magic pills. They just want you hooked on $17k/month vials. I’m not buying it.
Grace Rehman
January 4, 2026 AT 06:12They call it progress but it’s just a never-ending ladder of drugs and side effects. We’re treating symptoms with more chemicals while ignoring why the body breaks down in the first place. Maybe the real question isn’t how to kill cancer faster… but how to stop it from forming at all
Jerry Peterson
January 5, 2026 AT 08:45Coming from Nigeria, I’m amazed at how far treatment has come. But I also know that most people here never even get tested for HER2 status. If this knowledge could be shared with clinics in Lagos or Kano, imagine how many lives could be saved. This isn’t just science-it’s justice.